Facts About Fire
Fire Resistance Strength Facts!
Fire – Solid Timber is resistant to fire. It burns at a slow rate, and doesn’t melt or suddenly collapse as steel can when heated. Fire needs air to burn and because solid timber has no middle air cavity like a conventional framed building, timber has to burn from the outside in.
Resistance – When solid timber is exposed to high temperatures it will burn and begin to decompose to provide an insulating surface layer of char that retards further degradation of the wood. The rate of char on the outside of the timber log is initially fast but as the depth of char increases, the rate of char slows because of the increasing insulation provided.
Strength – Although timber is a combustible material, when it burns, this surface layer of char is created which helps to protect and maintain the strength and structural integrity of the remaining unburned timber beneath. Engineered pine solid wood walls have a char rate of 0.6mm per minute.
Fact – Most building fires are started by heat sources that ignite materials such as furnishings, curtains, synthetic carpets which can be highly flammable and it is these materials that emit toxic fumes that most often threaten life and limb. The building structure is usually not the first material ignited.
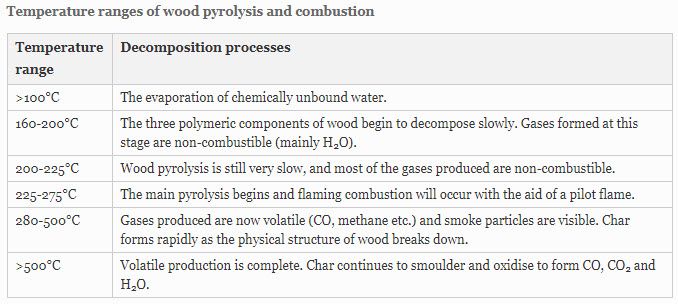
Science – Pyrolysis and Combustion
Solid timber is an insulator and a poor conductor of heat and electricity. Wood is a much better insulator than steel, concrete and glass.
When timber is progressively heated at raised temperatures, changes begin to occur in its structure, accelerated by further increase in temperature. The three polymeric components in the timber begin to thermally decompose to a mixture of volatile gases, tar (levoglucosan) and carbonaceous char. The decomposition is often regarded as the superposition of the individual constituent’s decomposition mechanisms: hemicellulose decomposes first [180 – 350°C] followed by cellulose [275 – 350°C], and lignin [250 – 500°C] The thermal stability of lignin is considered to be due to its heavily cross-linked structure and high molecular weight.